ORGANIC BUILDING BLOCKS
Organic building block is a term in organic chemistry, which is used to describe an organic compound with functional groups. Organic building blocks are mainly used for bottom-up modular assembly of metal-organic frameworks, organic molecular constructs, and supra-molecular complexes. Using organic building blocks ensures strict control of what a final compound will be.
US chemfine can offer a wide variety of organic building blocks including alkanes, alkenes, alkynes, arenes, arsenic compounds, carbonyl compounds, halogenated hydrocarbons, nitrogen compounds, organic phosphine compounds, organic silicon compounds, oxygen compounds and selenium compounds.
Applications
Organic building blocks are used in many fields as follow:
- Medicinal chemistry: In medicinal chemistry, organic building blocks are organic functionalized molecules that selected for the use in modular synthesis of novel drug candidates. And organic building blocks should be either mono-functionalised or possessing selectively chemically addressable functional groups so that they can be practically useful for the modular drug or drug candidate assembly. One of the major strategies for pharmaceutical industry involved in drug discovery is the use of organic building blocks prepared for fast and reliable constructions of small molecular compounds for biological screening. Usually, one-step synthesis of compounds from building blocks is, in most cases, faster and more reliable than multistep synthesis of target compounds.
- Organic chemistry: Organic building blocks are the basic components for organic synthesis. For example, fluoromalonate esters are versatile fluorinated building blocks that can be used for the introduction of fluorine atoms into aliphatic and aromatic heterocyclic systems. The study on syntheses of fluoromalonate compounds using commercially available fluorinating reagents on research and manufacturing scales is described, and the use of fluoromalonates for appropriate alkylation, acylation, Michael addition, annelation, and biotransformation processes is also presented, providing access to the synthesis of novel selectively fluorinated structures (Fig.1).
- Material chemistry: Utilizing organic building blocks is an effective strategy to synthesize materials. Simple molecule structures such as etraphenylethene (TPE) is an archetypal luminogen that are widely used in the construction of luminogenic materials and in the research and development of new organic light emitting diodes (OLEDs), solid-state lasers, photoconductive and photovoltaic devices and nonlinear optics materials. The simplicity of manufacture, arising from the fact that organic building blocks are processable either as the starting materials or as a precursor, thus allowing the possibility of tuning the material’s optical and electronic properties by using the enormous versatility of organic chemistry, has allowed the different applications of practical relevance.
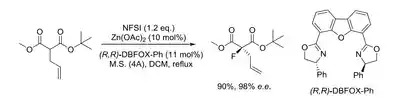
Fig. 1. Synthesis of fluoromalonates using N-fluorobenzenesulfonimide (NFSI)
Alkanes
In organic chemistry, alkanes are acyclic saturated hydrocarbons. In other words, the alkanes are compounds that hydrogen and carbon atoms arranged in a tree structure and in which all of carbon-carbon bonds are single. In alkanes, there are four bonds per carbon atom (C-C or C-H). The longest series of linked carbon atoms in a molecule is called a carbon backbone or a carbon skeleton. They can be thought of as molecular trees on which more active/reactive functional groups of biological molecules can be suspended. There are two main commercial sources of alkanes: petroleum (crude oil) and natural gas.
US chemfine provides different kinds of alkanes with optimal prices for our customers.
Applications
Industry: Propane is a popular choice for barbecues and portable stoves because the low boiling point makes it vaporize once it is released from pressurized containers. And because it can be easily transported, it is a common fuel for domestic heat and backup electrical generation in sparsely populated areas without natural gas pipelines. N-Butane can be used as a fuel gas, a perfume extraction solvent, and as a raw material for the production of ethylene and butadiene, which are the main components of a synthetic rubber. When mixed with propane and other hydrocarbons, it may be commercially known as liquefied petroleum gas. It is used as a gasoline component, as a feedstock for the production of petrochemical products, as a fuel for cigarette lighters, and as a propellant for aerosol sprays such as deodorants.
Synthetic chemistry: Heptane (and its isomers) is widely used in the laboratory as a non-polar solvent. As a liquid, it is very suitable for transportation and storage. In the fat spot test, heptane is used to dissolve the oil spots to show the previous presence of organic compounds on a stained paper. This is done by shaking the dyed paper in heptane solution for about half a minute. Petroleum ether is mainly used as a solvent for the extraction of oils or as a solvent and developing solvent for chromatographic analysis.
Medical chemistry: The main component of liquid paraffin is a mixture of alkanes. It is insoluble in water and alcohol, soluble in ether and chloroform and also cannot be absorbed in body. In medicine, liquid paraffin is used in the nasal drops and, also in the intestinal lubrication laxatives.
Alkanes
In organic chemistry, alkenes are unsaturated hydrocarbons containing at least one carbon-carbon double bond. The simplest alkene is ethylene (C2H4), which is named by the International Union of Pure and Applied Chemistry (IUPAC), is the organic compounds produced on the largest scale industrially. The physical properties of alkenes depend on the molecular mass. The simple alkenes such as ethylene, propylene and butene are gases at room temperature, linear alkenes containing five to sixteen carbon atoms are liquid, and higher alkenes are usually waxy solids.
Alkenes are non-polar compounds, in which the only intermolecular force is the dispersion force, so the alkenes are not soluble in water but soluble in carbon tetrachloride and other organic solvents. The chemical property of alkenes is relatively stable, but more active than alkanes. Considering that the carbon-carbon double bonds in alkenes are stronger than the carbon-carbon single bonds in alkanes, most of the alkenes react by breaking the double bonds and forming two new single bonds.
Alkenes are known as the raw materials in petrochemical industry because they can be applied in a wide range of reactions including addition reaction, polymerization reaction and metal complexation reaction.
Applications
- Addition reaction: Hydrogenation of alkenes produces the corresponding alkanes. The reaction is carried out at a temperature of 200 °C in the presence of a metal catalyst. Common industrial catalysts are platinum, nickel or palladium catalysts. In the presence of a suitable photosensitizer, the alkene can react with reactive oxygen species such as hydroxyl radicals, singlet oxygen or superoxide ion produced by the photosensitizer. A common example is the [4+2]-cyclicaddition of singlet oxygen to a diene to yield an endoperoxide.
- Polymerization reaction: Polymerization of alkenes produces high-value polymers such as plastic polyethylene and polypropylene. Polymers derived from alkene monomers are generally referred to as polyolefins. A polymer from alpha-olefins is called a polyalphaolefin (PAO). Polymerization can proceed via free radical or ionic mechanisms, converting double bonds into single bonds and forming single bonds to link other monomers.
- Metal complexation: Alkenes are important ligands in transition metal alkene complexes. Two carbon centers bonded to the metal are using C-C pi- and pi*-orbitals. Mono- and diolefins are often used as ligands in stable complexes. Cyclooctadiene and norbornadiene are popular chelating agents, and even ethylene is sometimes used as a ligand, for example in Zeise’s salt. In addition, metal-alkene complexes are intermediates in many metal-catalyzed reactions, including hydrogenation, hydroformylation and polymerization.
Alkanes

Alkynes are unsaturated hydrocarbons containing at least one carbon-carbon triple bond in organic chemistry. Alkynes are generally hydrophobic like other hydrocarbons, but tend to be more reactive. Alkynes are more unsaturated than alkenes, but in some reactions, alkynes are less reactive than alkenes. For example, in a molecule having one alkenyl and one alkynyl group, addition occurs preferentially to alkenyl group. Simple alkyne compounds include acetylene, propyne and so on. Among them, acetylene is the most important alkyne, which can be used to weld and cut metal in the industry (oxyacetylene flame), and is also the basic raw material for manufacturing acetaldehyde, acetic acid, benzene, synthetic rubber and synthetic fiber.
Applications
- Organic chemistry: Alkynes are “doubly unsaturated,” which means that 2 equivalents of halogen or related HX reagent (X = halide, pseudohalide, and so on) can be added into per alkyne unit. Alkynes can undergo different cycloaddition reactions. The most notable reaction is the Diels-Alder reaction with 1,3-dienes to produce 1,4-cyclohexadiene. This general reaction has been widely developed, and electrophilic alkynes are particularly effective dienophiles. The cycloadduct obtained from the addition of alkynes to 2-pyrone eliminates carbon dioxide and gives an aromatic compound. Other specialized cycloaddition reactions include multi-component reactions such as alkyne trimerization to produce aromatics and [2+2+1]-cyclicaddition of alkynes, alkenes and carbon monoxide in the Pauson-Khand reaction. Cycloadditions involving alkynes are usually catalyzed by metals.
- Nature product: In 1826, the first naturally occuring acetylenic compound, dehydromatricaria ester, was isolated from Artemisia species. Polyyne is a subset of this type of natural product that has been isolated from cultures of a wide variety of plant species, higher fungi, bacteria, marine sponges and corals. Some acids, such as tylonic acid, contain alkynyl groups. Other examples are cicutoxin, oantantoxin, falcarinol and carotatoxin, which are highly bioactive such as nematicides.
- Medical chemistry: Alkynes appear in some medicines, including the noretynodrel pill. Carbon-carbon triple bonds exist in marketed drugs such as the antiretroviral efavirenz and the antifungal terbinafine. The molecule called ene-diyne is characterized as a ring containing alkane between two alkyne groups. These compounds, such as calicheamicin, are known as the most aggressive antitumor drugs, so that ene-diyne is sometimes referred to as a “warhead”. The ene-diyne rearranges through Bergman’s cyclization, resulting in a highly active free-radical intermediate that attacks the tumor’s DNA.
Amino Acids
Amino acids are important organic components of proteins, which are essential macromolecules in all living organisms. They play vital roles in a variety of biological processes, including enzyme function, cell signaling, structural support, and molecular transport. There are 20 standard amino acids commonly found in proteins. These amino acids are distinguished by their side chains (R groups), giving each amino acid unique chemical properties.
US chemfine offers its customers a wide range of high-quality amino acids. Whether you are a researcher, scientist or health enthusiast, we offer a range of amino acids to meet your specific needs.
Explore our range below:
US chemfine offers the following standard amino acids:
- Glycine (Gly)
- Alanine (Ala)
- Valine (Val)
- Leucine (Leu)
- Isoleucine (Ile)
- Glutamic acid (Glu)
- Histidine (His)
- Tryptophan (Trp)
- Serine (Ser)
- Threonine (Thr)
- Cysteine (Cys)
- Asparagine (Asn)
- Aspartic acid (Asp)
- Methionine (Met)
- Phenylalanine (Phe)
- Tyrosine (Tyr)
- Glutamine (Gln)
- Lysine (Lys)
- Arginine (Arg)
Unusual Amino Acids Available from US chemfine
Non-standard amino acids are “non-proteinogenic” because they are not genetically encoded or incorporated during translation. Non-natural amino acids can enhance the stability or functionality of a therapeutic target and can be site-specifically incorporated into synthetic custom peptides.
US chemfine offers non-standard amino acids, including analogs, modified amino acids, specially functionalized structural units, polyethylene glycolated amino acids, and more, suitable for various research applications, including post-translational modifications.
Amino acid analogs
Functional unnatural amino acids
• Alanine, glycine, valine and leucine analogs
• Arginine and lysine analogs
• Aspartic acid and glutamate analogues
• Cysteine and methionine analogs
• Phenylalanine and tyrosine analogs
• proline analogs
• Serine, threonine and statin analogs
• tryptophan analogs
• Azide and alkyne building blocks
• Acetylated amino acids
• Glycosylated amino acids
• Methylated amino acids
• Phosphorylated amino acids
• binding building blocks
• dye-labeled amino acids
• PEGylated amino acids and special linkers
• N-alpha-methylamino acid
• β-amino acid
• Heterocyclic amino acids
Applications:
- Protein research: amino acids are essential for studying protein structure, function, and interactions.
- Biotechnology: amino acids are used in the production of recombinant proteins, antibodies, and enzymes.
- Nutritional supplements: amino acids are used to formulate dietary supplements to support muscle growth, recovery, and overall health.
- Pharmaceuticals: Amino acids play a vital role in the development of drugs and therapies.
- Cosmetics and Personal Care: Certain amino acids are added to skin and hair care products for their beneficial properties.
Browse our range of organic building blocks/amino acids today and unlock the potential of these important molecular components!
Arenes

Arenes are the benzene and its derivatives that refer to hydrocarbons own one or more benzene rings. The origin name is formed in the early organic development, because this class of compounds is almost always found in volatile, scented substances, such as: benzoic acid from benzoin gum, benzaldehyde obtained from bitter almond oil. But many of aromatic compounds are not fragrant, so today’s arenes are compounds containing benzene ring. The most simple and most important arenes are benzene and its homologues toluene, xylene, ethylbenzene and so on.
Applications
Organic chemistry: Arenes are reactants in many organic reactions. In a coupling reaction, the metal catalyzes the coupling between two types of free radical segments. Common coupling reactions result in the formation of new carbon-carbon bonds such as alkylaromatics, vinylaromatics, carbon-carbon double bonds, carbon-nitrogen bonds (anilines) or new carbon-oxygen bonds (aryloxy compounds).
Fig.1. Suzuki coupling.
Medical chemistry: Salicylic acid is one of the most famous arenes. In ancient times, salicylic acid has been not only used to relieve pain and fever, but also have anti-inflammatory effect. In modern medicine, methyl salicylate is also used to relieve joint and muscle pain; salicylic acid choline is widely used to treat oral ulcers. Salicylic acid is also a key exfoliating ingredient in many skin care products and is used in the treatment of acne, seborrheic dermatitis, psoriasis, corns and keratosis. Topical salicylic acid has antibacterial properties against the microbes, and its corrosion resistance is close to that of phenol.
Industry: The biggest use of benzene is to prepare the styrene, and polystyrene can be obtained by the polymerization of styrene. Polystyrene has excellent electrical properties, with well heat resistance and low price, and has become one of the most commonly used general-purpose plastics today. It is commonly used as shockproof packaging materials. Followed by hydrogenation of benzene into cyclohexane, cyclohexane is the raw materials to produce nylon. The use of nylon includes making fibers and engineering plastics, the fibers can be woven into a variety of fabrics and can also be made into the cords of aircraft and automobile tire. Industrial xylenes are a mixture of three xylene isomers (ortho, meta and para) and ethylbenzene. Among them, para-xylene can be made into terephthalic acid at high temperature, which is the main raw material for synthesizing polyester resin (polyester). Anthracene and naphthalene are polycyclic aromatic hydrocarbons, which are important raw materials for the production of synthetic dyes and pharmaceuticals.
Arsenic Compounds
Organic arsenic compounds are chemical compounds that contain arsenic-carbon bonds. Some organic arsenic compounds, also known as “organoarsenicals “, are used industrially as insecticides, herbicides and fungicides. Despite the toxicity, arsenic compounds are well known. Organoarsenic chemistry has played a prominent role in the history of chemistry. The oldest known organic arsenic compound, cacodyl, is sometimes classified as the first synthetic organometallic compound. Salvarsan is one of the earliest drugs and earn a Nobel Prize for Paul Ehrlich. Various other arsenic compounds have also formerly been used
as antibotics or have other medical uses.
Applications
- Medical chemistry: Arsphenamine, also known as Salvarsan, which was introduced in the early 1910s, is the first effective treatment for syphilis because it is toxic to the bacterium Treponema pallidum. It is also used to treat trypanosomiasis. This arsenic compound is the first modern chemotherapeutic agent. The project about Arsphenamine is the first organized team effort to optimize the bioactivity of lead compounds through systematic chemical modifications, which is the basis of modern pharmaceutical research. Neosalvarsan is a synthetic chemotherapeutic agent and an organic arsenic compound. It was launched in 1912 and replaced the more toxic and less water-soluble Arsphenamine as an effective treatment for syphilis. Arsenicin A is a naturally occurring organoarsenic compound that isolated from the marine sponge Echinochalina bargibanti. The compound possesses a cage-like structure similar to adamantane in which the four methanetriyl carbon bridgeheads and three of the six methylene bridges are replaced by arsenic atoms and oxygen atoms, respectively. Arsenicin A is active against promelocytic leukemia cells at lower concentrations than the drug Trisenox.
- Agricultural chemistry: Arsanilic acid, also known as aminophenyl arsenate, is an organic arsenic compound which is an amino derivative of phenylarsonic acid with an amine group at the 4-position. It is a crystalline powder that was introduced medically as Atoxyl in the late 19th century and its sodium salt was used by injection at the beginning of the 20th century. Arsanilic acid has been used as a veterinary feed additive to promote growth and prevent or treat dysentery in poultry and swine for a long time. Methylarsonic acid is an organic arsenic compound of the formula CH3AsO3H2. It is a colorless, water-soluble solid. The salts of such compounds, such as disodium cacodylate, have been widely used as herbicides and fungicides in the cultivation of cotton and rice.
Oxygen Compounds

Oxygen is a chemical element with the symbol O and the atomic number of 8. It is a member of the chalcogen on the periodic table and a highly active non-metallic element. Oxygen can be used for cellular respiration. The major classes of organic molecules in the organism contain oxygen, such as proteins, nucleic acids, carbohydrates and fats, as well as inorganic compounds, the major component of animal skins, teeth and bones.
US chemfine can offer a range of competitive oxygen compounds.
Applications
- Industry: Alcohol is organic compound with a hydroxyl functional group (-OH) bound to a saturated carbon atom. Some alcohols, mainly ethanol and methanol, can be used as fuel. After the turbocharger or supercharger has pressurized the air, the performance of the fuel in the forced-induction internal combustion engine can be increased by injecting the alcohol into the intake port. This cools the pressurized air, providing a denser air charge, which allows more fuel and therefore more power. Pentanol is used as a solvent for coating CDs and DVDs and it has all the properties necessary to replace gasoline as an internal combustion fuel.
- Medical chemistry: Isoetarine is a selective short-acting β2 adrenergic receptor agonist. In the β2 agonist family, it is called “granddaughter of adrenalin” and can rapidly relieve bronchospasm and asthma. Epinephrine is the first, followed by isoproterenol. Isoetharine is the third drug in this line, the third generation of the original. Ethanol is used as an antiseptic to disinfect the skin before injecting, usually with iodine. Alcohol-based soap is commonly used in restaurants and is convenient for there is no need to dry the soap because of the volatility of the alcohol. Alcohol-based gel has become common as hand lotion.
- Organic chemistry: An orthoester is a functional group containing three alkoxy groups attached to one carbon atom. Orthoesters are usually considered as products of exhaustive alkylation of unstable orthocarboxylic acids. An typical example is ethyl orthoacetate, more correctly known as 1,1,1-triethoxyethane. Orthoesters are used in organic synthesis as protecting groups for esters. Both triethylorthoacetate and trimethylorthoacetate are commonly used reagents with protecting groups in organic chemistry. Another example is the OBO protecting group (4-methyl-2,6,7-trioxa-bicyclo[2.2.2]octan-1-yl) which was developed by Elias James Corey and is formed by the action of (3-methyloxetan-3-yl)methanol on activated carboxylic acids in the presence of Lewis acids. The group is base stable and can be cleaved within two steps under mild conditions, mildly acidic hydrolysis yields the ester of tris(hydroxymethyl)ethane which is then cleaved using an aqueous carbonate solution.